| NDOH 03-2023/2024 | Appointment of a service provider to render occupational health services at Dr AB Xuma building & MBOD for a period of three (03) years
Compulsory Briefing session
Date: 29 February 2024
Time: 10:00am
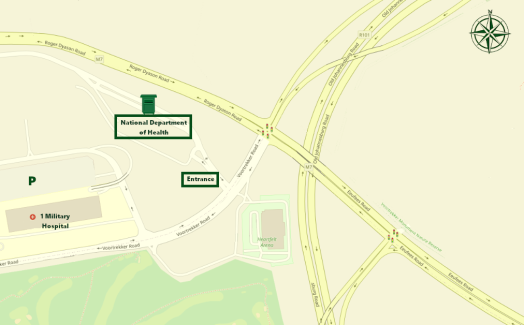
Venue: National Department of Health, Dr AB Xuma Building, 1112 Voortrekker Road, Thaba Tshwane, Pretoria
- Pricing Schedule
- Pricing Schedule SBD 3.3
- Addendum
- Bid Questions
| 19 February 2024 | 25 March 2024
Closing Date Extended | Enquiries:
Email: tenders@health.gov.za
Bids Received
|
| NDOH 35-2023/2024 | Supply and delivery of point of care testing devices, software and related consumables to the Department of Health for Non-Communicable Diseases and Primary Health Care for a period of three (3) years
Non-Compulsory Virtual Briefing session
Date: 31 January 2024
Time: 10:00am
Register for virtual briefing section
- Annexure A
- POC Pricing Schedule
* Cover page Closing Date Extension ↓
- Addendum 1
- SCM-Bid documents SBD 3.2 (16)
- Questions from Bidders
- Annexure A Erratum 1
Inclusion of Contractual Price Adjustments
| 19 January 2024 | 29 February 2024 @11:00 | Enquiries:
Email: tenders@health.gov.za
Bids Received |
| NDOH 49-2023/2024 | Appointment of a project management team for the management of medico-legal matters in the provinces for a period of three (3) years.
Compulsory virtual briefing session will be held:
Date: 11 April 2024
Time: 10:00am
Online: Register for virtual briefing section
- Pricing Schedule
- Notice to bidders
- Extension Notice
| 12 March 2024 | 23 April 2024 @11:00
Closing Date Extended | Enquiries:
Email: tenders@health.gov.za
|
| NDOH 28-2023/2024 | Appointment of a service provider to supply, deliver and distribute Basic Life Support (BLS) essential equipment and training manuals for the National Department of Health.
There will be no briefing session | 24 November 2023 | 14 December 2023 @11:00 | Enquiries:
Email: tenders@health.gov.za
Bids Received ↓ |
| NDOH 06-2023/2024 | Appointment of a service provider for on demand printing and delivery of CCMDD material.
Cover Gauteng
Prescription Pads Gauteng
Medicine Collection Card
Questions and Answers
| 26 October 2023 | 20 November 2023 @11:00 | Enquiries:
Email: tenders@health.gov.za
Bids Received
Award Notice |
| DOH 745-2022/2023 | Request for quotation for the supplying, assembling, testing, training and commissioning of an Amplitude Interated Electroencephalograph Unit -(aEEG) at Tshilidzini regional hospital Vhembe, Thohoyandou, Limpopo
| 18 September 2023 | 26 September 2023 @12:00 | Enquiries:
Email: tenders@health.gov.za
|
| NDOH 43-2023/2024 | Appointment of a service provider for the continued hosting, maintenance, support and enhancement of the existing ideal health facility information system for the National Department of Health for a period of three (3) years.
Compulsory briefing session will be held:
Date: 19 October 2023
Time: 11:30AM
Venue: 1112 Voortrekker Road, Dr AB Xuma Building, Thaba Tshwane, Pretoria
| 5 October 2023 | 30 October 2023 | Enquiries:
Email: tenders@health.gov.za
Bids Received ↓ |
| NDOH 27-2023/2024 | Appointment of a service provider for the continued hosting, maintenance, support and enhancement of the existing Community Health Worker and Outreach Team Leader database for the National Department of Health for a period of three (3) years.
Compulsory briefing session will be held:
Date: 10 November 2023
Time: 11:00AM
Venue: 1112 Voortrekker Road, Dr AB Xuma Building, Thaba Tshwane, Pretoria
- Pricing Schedule
| 02 November 2023 | 08 Decenber 2023 @11:00 | Enquiries:
Email: tenders@health.gov.za
Bids Received ↓ |
| NDOH 36-2023/2024 | Appointment of a service provider/s to render services for Public Health Sector: Multipronged Mass Media Strategic Communication including National Health Insurance (NHI) & Health and Wellness Communication Campaign
Compulsory briefing session will be held:
Date: 11 September 2023
Time: 10:00AM
Venue: 1112 Voortrekker Road, Dr AB Xuma Building, Thaba Tshwane, Pretoria
- Pricing Wellness Campaigns and Events
- SBD 3.3 Strategic Communication Pricing schedule
- Addendum 02
- SBD 3.3 Campaigns Pricing schedule
- SBD 3.3 Events Pricing schedule
| 29 August 2023 | 02 October 2023 @11:00 | Enquiries:
Email: tenders@health.gov.za
Bids Received ↓ |
| DOH 387-2023/2024 | Request for quotation for procurement of Label Printer (406)- ZD230-T Z1AE-ZD2X-3C0, three (3) years; Comprehensive collect repair and return warranty for three (3) years
| 29 August 2023 | 07 September 2023 @11:00 | Enquiries:
Email: tenders@health.gov.za
|
| DOH 386-2023/2024 | Request for quotation for procurement of Label Printer (406)- ZD230-T Z1AE-ZD2X-3C0, three (3) years; Comprehensive collect repair and return warranty for three (3) years
| 29 August 2023 | 04 September 2023 @11:00 | Enquiries:
Email: tenders@health.gov.za
|
| DOH 385-2023/2024 | Request for quotation for procurement of Label Printer (406)- ZD230-T Z1AE-ZD2X-3C0, three (3) years; Comprehensive collect repair and return warranty for three (3) years
| 29 August 2023 | 06 September 2023 @11:00 | Enquiries:
Email: tenders@health.gov.za
|
| DOH 383-2023/2024 | Request for quotation for procurement of Label Printer (406)- ZD230-T Z1AE-ZD2X-3C0, three (3) years; Comprehensive collect repair and return warranty for three (3) years
| 31 August 2023 | 08 September 2023 @11:00 | Enquiries:
Email: tenders@health.gov.za
|
| DOH 382-2023/2024 | Request for quotation of procurement of uninterrupted power supply (UPS)- Eaton (145) ELP1600IEC- H with three (3) years
| 29 August 2023 | 04 September 2023 @11:00 | Enquiries:
Email: tenders@health.gov.za
|
| DOH 381-2023/2024 | Request for quotation of procurement of uninterrupted power supply (UPS)- Eaton (145) ELP1600IEC- H with three (3) years
| 29 August 2023 | 07 September 2023 @11:00 | Enquiries:
Email: tenders@health.gov.za
|
| DOH 380-2023/2024 | Request for quotation of procurement of uninterrupted power supply (UPS)- Eaton (145) ELP1600IEC- H with three (3) years
| 31 August 2023 | 08 September 2023 @11:00 | Enquiries:
Email: tenders@health.gov.za
|
| DOH 379-2023/2024 | Request for quotation of procurement of uninterrupted power supply (UPS)- Eaton (145) ELP1600IEC- H with three (3) years
| 29 August 2023 | 07 September 2023 @12:00 | Enquiries:
Email: tenders@health.gov.za
|
| DOH 378-2023/2024 | Request for quotation for procurement of servers
| 29 August 2023 | 06 September 2023 @11:00 | Enquiries:
Email: tenders@health.gov.za
|
| DOH 377-2023/2024 | Request for quotation for procurement of servers
| 31 August 2023 | 07 September 2023 @11:00 | Enquiries:
Email: tenders@health.gov.za
|
| DOH 376-2023/2024 | Request for quotation for procurement of servers
| 29 August 2023 | 05 September 2023 @11:00 | Enquiries:
Email: tenders@health.gov.za
|
| DOH 375-2023/2024 | Request for quotation for procurement of servers
| 31 August 2023 | 07 September 2023 @11:00 | Enquiries:
Email: tenders@health.gov.za
|
| DOH 373-2023/2024 | Request for quotation for procurement of servers
| 29 August 2023 | 06 September 2023 @12:00 | Enquiries:
Email: tenders@health.gov.za
|
| DOH 372-2023/2024 | Request for quotation for procurement of MFCL 5700 printers
| 29 August 2023 | 08 September 2023 @11:00 | Enquiries:
Email: tenders@health.gov.za
|
| DOH 371-2023/2024 | Request for quotation for procurement of MFCL 5700 printers
| 19 September 2023 | 22 September 2023 @12:00 | Enquiries:
Email: tenders@health.gov.za
Bids Received ↓ |
| DOH 370-2023/2024 | Request for quotation for procurement of desktop computers
| 31 August 2023 | 08 September 2023 @11:00 | Enquiries:
Email: tenders@health.gov.za
|
| DOH 369-2023/2024 | Request for quotation for procurement of desktop computers
| 29 August 2023 | 06 September 2023 @11:00 | Enquiries:
Email: tenders@health.gov.za
|
| DOH 367-2023/2024 | Request for quotation for procurement of desktop computers
| 29 August 2023 | 07 September 2023 @12:00 | Enquiries:
Email: tenders@health.gov.za
|
| DOH 366-2023/2024 | Request for quotation for procurement of desktop computers
| 29 August 2023 | 07 September 2023 @12:00 | Enquiries:
Email: tenders@health.gov.za
|
| DOH 365-2023/2024 | Request for quotation for procurement of MFCL 5700 printers
| 29 August 2023 | 06 September 2023 @12:00 | Enquiries:
Email: tenders@health.gov.za
|
| NDOH 37-2023/2024 | The establishment of a panel of registered medical practitioners for the certification and review committees at the medical bureau for occupational diseases (MBOD) for a period of five (5) years
Non-compulsory briefing session date and time: 25 Sugust 2023 at 11:00
Location: 144 de Korte street, Braamfontein, Johannesburg, 2000
Physical submission location: 1112 Voortrekker road, Dr AB Xuma building, Thaba Tswane, Pretoria, 0187
| 18 August 2023 | 08 September 2023 | Enquiries:
Email: tenders@health.gov.za
Bids Received ↓
Award Notice
|
| NDOH 15-2023/2024 | Appointment of Service Provider(s) to Supply and Deliver of Various Warehouse Items for a Contract Period of Three (3) Years
No briefing session
- Costing Model-Excel Spreadsheet
| 11 August 2023 | 01 September 2023 | Enquiries:
Email: tenders@health.gov.za
Bid Opening Certificate |
| NDOH 09-2023/2024 | Invitation for call for proposals to Non-Governmental Organisations (NGO’s) for Health System Research grant for a period of 36 months (3 years) ↓
There will be no briefing session
| 14 July 2023
| 7 August 2023 @11:00 | Enquiries:
Email: tenders@health.gov.za
Bids Received ↓
Award Notice |
| NDOH 10-2023/2024 | Appointment of Multiple Internet Service Providers (ISPs) to facilitate direct internet access for the connectivity of 800 National Department of Health (NDoH) facilities nationwide for a period of 36 months (3 years) ↓
Final Health identified sites June 2023 ↓
Compulsory briefing session: 24 July 2023 at 11:00
Venue: Dr AB Xuma Building, 1112 Voortrekker Road, Thaba Tshwane, Pretoria
List of Questions and response ↓
* New Cover Page ↓
* Extention Notice ↓ | 12 July 2023
| New Date
14 August 2023 @11:00 | Enquiries:
Email: tenders@health.gov.za
Bids Received ↓
Award Notice ↓ |
| NDOH 30-2023/2024 | Appointment of a service provider to conduct quality assessment of food service units in 149 hospitals. ↓
There will be no Compulsory Briefing Session for this bid
| 5 October 2023
| 30 October 2023 | Enquiries:
Email: tenders@health.gov.za
Bid Pricing Schedule ↓
Bids Received ↓ |
| NDOH 34-2023/2024 | Appointment of service provider for the provision of monitoring and evaluation on the Indoor Residual Spraying (IRS) programme in selected districts in Southern Mozambique ↓
There will be no Compulsory Briefing Session for this bid
| 6 July 2023
| 31 July 2023 | Enquiries:
Email: tenders@health.gov.za
Bids Received ↓
Award notice ↓ |
| NDOH 16-2023/2024 | Appointment of service provider for the implementation of the indoor residual spraying (IRS) programme in selected districts in southern Mozambique ↓
There will be no Compulsory Briefing Session for this bid
| 6 July 2023
| 31 July 2023 | Enquiries:
Email: tenders@health.gov.za
Bids Received ↓
Award notice ↓ |
| NDOH 12-2023/2024 | Appointment of service provider for hosting, maintenance, support, and enhancement of the existing district health information system for the National Department of Health for a period of three (03) years ↓
There will be no Compulsory Briefing Session for this bid
| 21 June 2023 | 17 July 2023 | Enquiries:
Email: tenders@health.gov.za
Bids Received ↓
Award notice ↓ |
| NDOH 05/2023-2024 | Appointment of service provider for hosting, maintenance, support and enhancement of the existing internship and community service programme (ISCP) online system for the National Department of Health for a period of three (03) years ↓
There will be no Compulsory Briefing Session for this bid
| 21 June 2023 | 17 July 2023 | Enquiries:
Email: tenders@health.gov.za
Bids Received ↓
Award Notice ↓ |
| NDOH 31/2022-2023 | Appointment of a Professional Service Provider to provide Integrated Multi-pronged Strategic Communication Campaign for Public Health Policy Issues for a period of three (03) years. ↓
Addendum ↓
Compulsory briefing session: 17 February 2023 at 11:00
Venue: Dr AB Xuma Building, 1112 Voortrekker Road, Thaba Tshwane, Pretoria
| 6 February 2023 | 6 March 2023 | Enquiries:
Email: tenders@health.gov.za
Bids Received ↓
CANCELLATION NOTICE: TENDER NDOH 31-2022/2023 |
| NDOH 08/2023-2024 | Appointment of a service provider to manage, maintain, support and modernize the existing learning management system for the National Department of Health for a period of three (3) years. ↓
List of Questions and Responses ↓
| 29 May 2023 | 12 Junie 2023 | Enquiries:
Email: tenders@health.gov.za
Bids Received ↓
Award Notice |
| NDOH 08/2022-2023 | Appointment of a Professional Service Provider to provide Integrated Multi-pronged Health & Wellness Communication for a period of three (03) years. ↓
Addendum 1 ↓
Addendum 2 ↓
Bid documents SBD 3.3 ↓
Compulsory briefing session: 17 February 2023 at 10:00
Venue: Dr AB Xuma Building, 1112 Voortrekker Road, Thaba Tshwane, Pretoria
| 6 February 2023 | 6 March 2023 | Enquiries:
Email: tenders@health.gov.za
Bids Received ↓
CANCELLATION NOTICE: TENDER NDOH 08-2022/2023 |
| NDOH 24/2022-2023 | Procurement of electronic upper arm non-invasive blood pressure machines for adults. ↓
Non-Compulsory virtual briefing session: 25 November 2022 at 11:00
Distribution list for Tender BP Machines ↓
| 18 November 2022 | 09 December 2022 | Enquiries:
Email: tenders@health.gov.za
Bids Received ↓
Award Notice ↓
|
| NDOH 23/2022-2023 | Appointment of a service provider for hosting, maintenance, support and enhancement of the existing district health information system (DHIS) for the National Department of Health for a period of three (years). ↓
Compulsory virtual briefing session: 30 November 2022 at 11:00 | 22 November 2022 | 13 December 2022 | Enquiries:
Email: tenders@health.gov.za
Bids Received ↓
CANCELLATION NOTICE: TENDER NDOH 23/2022-2023 |
| BMZ 2012 65 198 | Republic of South Africa
International, open tender for HIV Prevention Program II – Support to the South African COVID-19 Vaccination Campaign
Tenderer: National Department of Health with DG Murray Trust (DGMT) as Project Executing Agency
- Invitation for Bids GTAI Procurement of Mobile Clinics
- RSA Tender – Mobile Clinics Publication
| | 24 October 2022, 16:00h South Africa time | |
| NDOH 29/2023-2024 | Appointment of a service provider to facilitate further rollout of the ideal clinic realization and maintenance (ICRM) programme for a period of three (3) years. ↓
Compulsory Briefing session Date: 19 October 2023 at 10:00
Venue: National Department of Health; Dr AB Xuma building; 1112 Voortrekker road, Thaba Tshwane, Pretoria.
| 05 October 2023 | 2 November 2023 | Enquiries:
Email: tenders@health.gov.za
Bids Received ↓ |
| NDOH 20/2022-2023 | Appointment of a service provider(s) to supply various warehouse items for a contract period of three (03) years. ↓
Compulsory Briefing session Date: 15 November 2022 at 11:00
Venue: National Department of Health; Dr AB Xuma building; 1112 Voortrekker road, Thaba Tshwane, Pretoria.
Costing Model-Excel 2022-2025 ↓ | 08 November 2022 | 29 November 2022 | Enquiries:
Email: tenders@health.gov.za
Bids Received ↓ |
| NDOH 19/2022-2023 | Appointment of a service provider to render security services to the National Department of health offices, Dr AB Xuma building and MBOD building for a period of three years. ↓
- Compulsory Briefing Session: 15 December 2022 at 10:00, Dr AB Xuma Building, 1112 Voortrekker road, Thaba Tshwane, Pretoria.
- PRICING SCHEDULE 2022 – 2023 [Excel document]
- PRICING SCHEDULE 2022 – 2023
- List of Questions and Responses
- Private Security Service addendum
| 07 December 2022 | 20 January 2023 | Enquiries:
Email: tenders@health.gov.za
Bids Received ↓
Award Notice ↓ |
| NDOH 18/2022-2023 | Appointment of a service provider for the transportation of cardio-respiratory organs (heart and lungs) of deceased miners and former miners in accordance with the Occupational Diseases in mines and works Act (78 of 1973) for a period of three (03) years. ↓
Compulsory Briefing session Date: 16 November 2022 at 11:00
Venue: Medical Bureau for Occupational Diseases (MBOD) 144 De Korte Street; Braamfontein; Johannesburg.
| 08 November 2022 | 29 November 2022 | Enquiries:
Email: tenders@health.gov.za
Bids Received ↓ |
| NDOH 16/2022-2023 | Appointment and accreditation of service providers to be listed on to the Medical Bureau of occupational Diseases (MBOD) service provider database to provide benefit medical examinations and postmortem services on behalf of the (MBOD) in all provinces and relevant SADC countries for a period of three (03) years. ↓
There will be no Compulsory Briefing Session for this bid
| 08 November 2022 | 29 November 2022 | Enquiries:
Email: tenders@health.gov.za
Bids Received ↓
|
| NDOH 08/2021-2022 | BID CANCELED
Appointment of a service provider for the delivery of security systems (operating lease of security systems) for the National Department of Health for a period of forty-eight (48) months from the date of appointment. ↓
Compulsory Briefing session date: 22 September 2021 @ 10:00
Venue: Dr AB Xuma Building,
Roger Dyson & Voortrekker Road,
Pretoria
Compulsory briefing session attendance register ↓ | 15 September 2021 | 7 October 2021 @ 11:00 | Enquiries:
Email: tenders@health.gov.za
Bids Received ↓
Cancellation notice |
| NDOH 07/2022-2023 | Appointment of a service provider for the provision of services for the central chronic medicine dispensing and distribution programme for public sector patients for a period of four (4) years. ↓
Compulsory Briefing Session:
Date: 23 August 2022
Time: 10:00-12:00
Ctrl+Click here to join the meeting
| 17 August 2022 | 7 September 2022 | Enquiries:
Email: tenders@health.gov.za
Bid Spec Response and Pricing Schedule Appendices A-D ↓
List of bids Received 2022-2023 ↓ |
| NDOH 04/2022-2023: | Appointment of a service provider for the supply and delivery of DDT Wettable powder for a period of three (03) years. ↓
| 27 May 2022 | 20 June 2022 | Enquiries:
Email: tenders@health.gov.za
Bids Received ↓
Award Notice ↓ |
| NDOH 03/2022-2023: | Appointment of a service provider to conduct an actuarial evaluation of the mines and works compensation fund. ↓
Compulsory Briefing Session/Site Inspection:
11 July 2022 at 11:00
MBOD
144 De Korte Street; Braamfontein; Johannesburg.
Addendum 01 signed ↓
| 01 July 2022 | 26 July 2022 | Enquiries:
Email: tenders@health.gov.za
List of Bids Received Actuarial Valuation of Mines ↓
Award Notice ↓ |
| NDOH 02/2022-2023 | Appointment of a service provider to assess the public mental health system’s ability to cater to the need of children and adolescents with psychosocial disabilities. ↓
Pricing Schedule ↓ | 12 May 2022 | 07 June 2022 | Enquiries:
Email: tenders@health.gov.za
Bids Received ↓
Award Notice ↓ |
| NDOH 01/2022-2023 | BID CANCELED
Appointment of a service provider to render physical security services to National Department of Health offices; DR AB Xuma building; for a period of thirty-six (months). ↓
Pricing Schedule(2) ↓
Compulsory Briefing Session/Site Inspection:
18 May 2022 at 10:00 am.
Dr AB Xuma building
1112 Voortrekker rd;
Pretoria; townlands 351-JR.
Pricing Schedule(Excel) ↓
List of bids received NDOH 01/2022-2023 Physical Security Services ↓ | 11 May 2022 | 06 June 2022 | Enquiries:
Email: tenders@health.gov.za
|
| NDOH 28/2021-2022 | Appointment of a service provider to supply; deliver and distribute basic life support (BLS) essential equipment and training manuals for the National Department of Health. ↓ | 01 February 2022 | 28 February 2022 at 11:00 am | Enquiries:
Email: tenders@health.gov.za
There will not be a briefing session
Bids Received ↓
|
| NDOH-26/2021-2022 | Appointment of a Service Provider for the Supply of the RDT in the Malaria Endemic Provinces for a period of two (2) years. ↓ | 13 December 2021 | 25 January 2022 at 11H00 | Enquiries:
Email: tenders@health.gov.za
Bids Received ↓
Award Notice ↓ |
| NDOH-25(2021/2022) | Supply and Delivery of DDT Wettable Powder to the National Department of Health for a period of three(03) years.. ↓ | 13 December 2021 | 17 January 2022 at 11H00 | Enquiries:
Email: tenders@health.gov.za
|
| NDOH-19(2021/2022) | Appointment of a Professional Service to Render the Reintegration Programme for the National Department of Health. ↓ | | 03 DECEMBER 2021 | Enquiries:
Email: tenders@health.gov.za
Bids Received ↓
Bid Results ↓ |
| NDOH-18(2021-2022) | Appointment of a Service Provider for Provision of Hygiene and Cleaning Services at the Medical Bureau for Occupational Diseases (MBOD) for a period of three (3 years). ↓
Pricing Schedule ↓
Compulsory Briefing Session/Site Inspection:
01 March 2022 at 11h00 am
MBDO:
144 De Korte Street
Braamfontein
Johannesburg | 09 February 2022 | 10 March 2022 at 11h00 am | Enquiries:
Email: tenders@health.gov.za
Bids Received ↓
Award Notice Katie M-Mathasani JV ↓ |
| NDOH 16/2021-2022 CCMDD | BID CANCELED
The Provision of Services for the Central Chronic Medicine Dispensing and Distribution Programme for Public Sector Patients. ↓
A virtual compulsory briefing session will be held:
Date: 25 October 2021
Time: 10:00 – 12:00
| 14 October 2021 | 19 November 2021 | Enquiries:
Email: tenders@health.gov.za
CCMDD Tender Briefing Presentation
CCMDD Virtual briefing session QA 25102021
CCMDD Response document
Bids Received
|
| NDOH 13/2021-2022 | Request for appointment to serve as a Sub-Recipient (SR) for the National Department of Health Global Fund TB/HIV program from April 2022-March 2025. ↓
Virtual Briefing Session (non-compulsory):
25 October 2021 at 09:00 am
| 11 October 2021 | 10 November 2021 (on or before 11:00) | Enquiries:
Email: tenders@health.gov.za
SCM – Tender Briefing Presentation
Terms of Reference – Tender Briefing Presentation
National TB Priorities – Briefing Session
List of Questions and Response
Bids Received
Award Notice ↓ |
| NDOH 12/2021-2022 | Appointment of a Service Provider for the Provision of Cleaning and Hygiene Services at the National Department of Health, Pretoria Head Office, 1112 Voortrekker Road, Pretoria Townlands 351-JR, Dr. AB Xuma Building, Pretoria, for a period of Three (3) Years ↓
Pricing Schedule ↓
| 27 AUGUST 2021 | 20 SEPTEMBER 2021,11:00 | Enquiries:
Email: tenders@health.gov.za
Bids Received ↓
Bids Results ↓
|
| NDOH 07/2021-2022 | Appointment of a printing company to print and deliver data collection tools for Community Health Workers in identified Health Districts ↓
| 28 June 2021 | 20 July 2021, 11:00 | Enquiries:
Email: tenders@health.gov.za
Bids Received ↓ |
| NDOH 03/2021-2022 | Bid Document: Appointment of a professional service provider for the rendering of travel management services for the National Department of Health for the period of three (03) years. ↓
Revised Pricing Schedule ↓
Technical Evaluation Scorecard ↓
Addendum: Closing Date Extension ↓ | 7 May 2021 | 08 June 2021, 11:00
Important Notice:
Kindly be advised that the closing date of this bid has been moved from 01 June 2021 to 08 June 2021 at 11:00AM
| Enquiries:
Email: tenders@health.gov.za
Public opening certificate↓
Bid Results ↓ |
| NDOH 02/2021-2022 | The appointment of an entity to provide quality assurance services for Covid-19 related Personal Protective Equipment (PPE) on behalf of the department of health for a period of twenty-four (24) months. ↓
Invitation To Bid ↓
Price schedule QA updated 29.04.2021 ↓
| 16 April 2021 | Important Notice:
Kindly be advised that the closing date of this bid has been moved from 07 May 2021 to 12 May 2021 at 11:00AM
| Enquiries:
Email: tenders@health.gov.za
|
|
2020 – 2021
|
|
|
|
| NDOH 48/2020-2021 | Appointment of a bidder to conduct an independent national survey on patient experience of care in public health organisations. ↓ | 12 February 2021 | 15 March 2021 | Enquiries:
Email: tenders@health.gov.za
|
| NDOH 40/2020-2021 | NDOH 40-2020-2021 Bid Award Notice (Deviation): Appointment of Project Portfolio Office (PPO) for the Services of Project Management Information Systems (PMIS) ↓
| 20 April 2021 | N/A | Enquiries:
Email: tenders@health.gov.za
|
| NDOH 34/2020-2021 | NOTICE OF TENDER CANCELLATION: Appointment of a professional service provider to render an Integrated multi-pronged health and wellness communication campaign for the National Department of Health (NDoH) for a period of three (3) years. | 12 April 2021 | N/A | Enquiries:
Email: tenders@health.gov.za
Tender Cancellation Letter ↓ |
| NDOH 35/2020-2021 | NOTICE OF TENDER CANCELLATION: Appointment of a professional service provider to render a strategic communication for the National Department of Health (NDoH) for a period of three (3) years.
| 12 April 2021 | N/A | Enquiries:
Email: tenders@health.gov.za
Tender Cancellation Letter ↓ |
| NDOH 22/2020-2021 | Appointment of service providers to be listed on the Medical Bureau of Occupational Diseases (MBOD) Service Provider Database to provide benefit examinations on behalf of the MBOD in all provinces and relevant SADC countries for a period of 3 years. ↓
| 4 September 2020 | 25 September 2020 | Enquiries:
Email: tenders@health.gov.za |
| NDOH 21/2020-2021 | Appointment of a Service Provider for the Transportation of Cardio-respiratory Organs of Deceased Mine Workers and Ex-mine Workers at the Occupational Health Cluster (MBOD/CCOD). ↓
| 28 August 2020 | 18 September 2020 | Enquiries:
Email: tenders@health.gov.za
Bids Received ↓
Erratum Notice ↓ |
| NDOH 14/2020-2021 | Appointment of a Third Party Logistics Service Provider for the Supply of Forwarding and Clearing Services for Personal Protective Equipment (PPE) Procured by the National Department of Health to be Centrally Held in a buffer Stock for a Period Of Six (6) Months. ↓
Delivery Points ↓
| 3 July 2020 | 16 July 2020 | Enquiries:
Email: tenders@health.gov.za
Bids Received↓ |
| NDOH 11/2020-2021 | Terms of reference for establishment of quality learning centres in line with National Health Quality Improvement Plan. ↓
| 24 July 2020 | 14 August 2020
@ 11:00 | Enquiries:
Email: tenders@health.gov.za
Bids Received ↓
|
| NDOH 07/2020-2021 | Supply and delivery of post-mortem blood alcohol sampling kits to forensic pathology kits to forensic pathology mortuaries for a period of two(2) years. ↓
| 16 October 2020 | 30 November 2020 | Enquiries:
Email: tenders@health.gov.za
Bids Received ↓
|
| NDOH 06/2020-2021 | Supply and delivery of toxicology sampling kits to forensic pathology kits to forensic pathology mortuaries for a period of three (3) years. ↓
Bid Response ↓
Annexure A ↓
Directors Categorisation [PBD9] ↓ | 8 May 2020 | 8 June 2020 | Enquiries:
Email: tenders@health.gov.za
Bids Received ↓ |
| NDOH 05/2020-2021 | Appointment of a bidder to conduct an independent national survey on patient experience of care in public health organisations. ↓
Bid Response ↓
| 22 May 2020 | 29 June 2020 | Enquiries:
Email: tenders@health.gov.za
|
| NDOH 01/2020-2021 | Appointment of a service provider for the continued development and enhancement of functionality of existing ideal health facility web-based information systems for the National Department of Health for a period of three (3) years. ↓
| 8 May 2020 | 8 June 2020 | Enquiries:
Email: tenders@health.gov.za
Bids Received ↓
|
| NDOHF02/2020-2021 | Refurbishment and upgrades at Oudtshoorn hospital and Oudtshoorn clinic in Western Cape Province, Eden District Municipality. | | | Public Opening Register ↓
|
| NDOHF07/2020-2021 | Refurbishment and upgrades at Pacaltsdorp and Parkdene clinics in Western Cape Province, Eden District Municipality | | | Public Opening Register ↓
|
|
2019 – 2020
|
|
|
|
| NDOH73/2019-2020 | Supply and Delivery of Tableau Software Licences to the National Department of Health: Affordable Medicines Directorate (AMD) Renewable for a Period of Three Years | Closed Bid
(not advertised in tender bulletin).
Distributed 9 April 2020 | 30 April 2020 | Enquiries:
Email: tenders@health.gov.za
Bids Received ↓
Contract Circular ↓
|
| NDOH 71/2019-2020 | Appointment of a service provider for the placement of advertisements in the print media and response handling for the National Department of Health for a period of three (3) years. ↓
| 20/03/2020 | Extended Closing date:
04 May 2020 | Enquiries:
Email: tenders@health.gov.za
Bids Received ↓
|
| NDOH 50/2019-2020 | Appointment of a service provider for malaria source reduction initiatives in Southern Mozambique. (Indoor residual spraying) ↓
| 30/03/2020 | Extended Closing date:
04 May 2020 | Enquiries:
Email: tenders@health.gov.za
Bids Received ↓
|
| NDOH 49/2019-2020 | Appointment of a Service Provider for the Provision of Administrative and Management Services to the National Department of Health (NDoH) TB/HIV Health Information System for a Period of Two (2) years. ↓
| 21 February 2020 | 13 March 202 | Enquiries:
Email: tenders@health.gov.za
|
| NDOH 46/2019-2020 | Supply, delivery, installation and commissioning of a bench-top high pressure ion chromatograph system consisting of a ion chromatograph (IC), an autosampler unit, a variable wavelength (or photodiode array) and chromatographic software (including a brother printer) ↓
| 13 December 2019 | 17 January 2020 | Enquiries:
Email: tenders@health.gov.za
Bids Received ↓
|
| NDOH 44/2019-2020 | Appointment of a bidder for the printing and distribution of maternity case records and the sick neonatal admission register. ↓
| 06 December 2019 | 17 January 2020 | Enquiries:
Email: tenders@health.gov.za
Bids Received ↓
|